
By: Ashley December 28,2019 November 11,2024
Gypsum, whose commercial value is of extreme importance, is widely used in society and associated with all industries. This article will focus on the introduction of gypsum definition, gypsum properties, types and applications.
Gypsum, also known as gypsum rock, is a kind of monoclinic mineral whose main chemical component is calcium sulfate (CaSO4). Hundreds of years ago, much of the earth was covered by oceans which were later surrounded by the mainland and then many inland dead seas were formed. As seawater evaporates and salts deposite, salt deposits such as halite, gypsum, liquid brine, petroleum, lime, clay, sandstone, and coal gradually formed. Over millions of years, these sedimentary combined with decayed vegetation and other ores, eventually forming stratified rocks, with gypsum deposits, the most common sulfates mineral in flat beds of about six to eight feet in thickness.
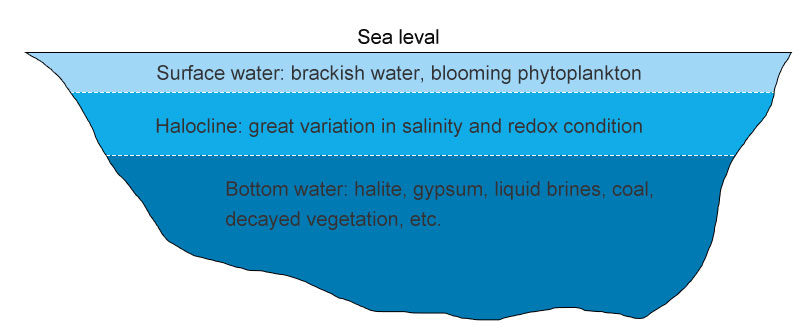
formation of gypsum
Gypsum is widely distributed and it is found in more than 90 countries and regions. The world’s gypsum reserves are about 2.4 billion tons, and the United States and Canada account for 49% of them. Britain, France, Mexico and Spain are other important localities of raw gypsum. The US consumes more than 30 million tons of gypsum each year. The properties of gypsum make it so popular all over the world. The following will introduce the characteristics of gypsum from several aspects.
| Another Name | raw gypsum |
| Chemical Classification | sulfate mineral |
| Color | white, colorless, as well as yellow or red when containing impurities |
| Luster | vitreous, silky or pearly |
| Transparency | transparent to translucent |
| Streak | white |
| Crystal System | monoclinic |
| Crystal Habit | tabular, bladed or blocky crystals with a slanted parallelogram outline |
| Cleavage | perfect |
| Fracture | conchoidal, sometimes fibrous |
| Mohs Hardness | 2 (can be scraped by nails) |
| Brittleness | flexural, but not self-bending |
| Mineral Density | 2.31 ~ 2.33 (light) |
| Form | bulk, saccharified texture when ruptured |
| Thermal Conductivity | very low |
There are three types of gypsum: raw gypsum, plaster of Paris and anhydrous gypsum. Gypsum powder usually said by masses refers to the powder of plaster, which can be solidified quickly when mixed with water. It can be used in building ornamental, gypsum line, gypsum board, ceramic mold, medical and so on.
The raw gypsum (chemical name: dihydrate calcium sulfate) with the chemical formula CaSO4·2H2O, is also known as dihydrate gypsum. Its theoretical components of CaO are 32.6%, SO3 46.5% and H2O 20.9%. Its Mohs hardness is 2 and density is 2.3 g/cm3.
Grind the raw gypsum into extremely fine powders, then heat the powder to 350° F and keep it for one to several hours. Most of the crystal water will be lost and it becomes white powdery gypsum finally, also known as hemihydrate gypsum (2CaSO4 · H2O), gypsum powder, bassanite or plaster. The Mohs hardness of the plaster of Paris is 2 and density is 2.55 ~ 2.67 g/cm3.
Due to insufficient water of plaster, a large amount of calcium ions and sulfate ions cannot be aligned and combined together in an orderly manner. At this time, if every three grams of plaster and 2 grams of water are fully mixed, water, playing a matchmaking role, will rearrange the original cluttered ions according to a certain rule and turn into crystal water of gypsum. Then, the plaster of Paris is turned into hard raw gypsum. This is the reason that the gypsum powder will gradually harden after adding water. In daily life, people use this feature of plaster to make plaster models, plaster statues, plaster bandages and building adhesives.
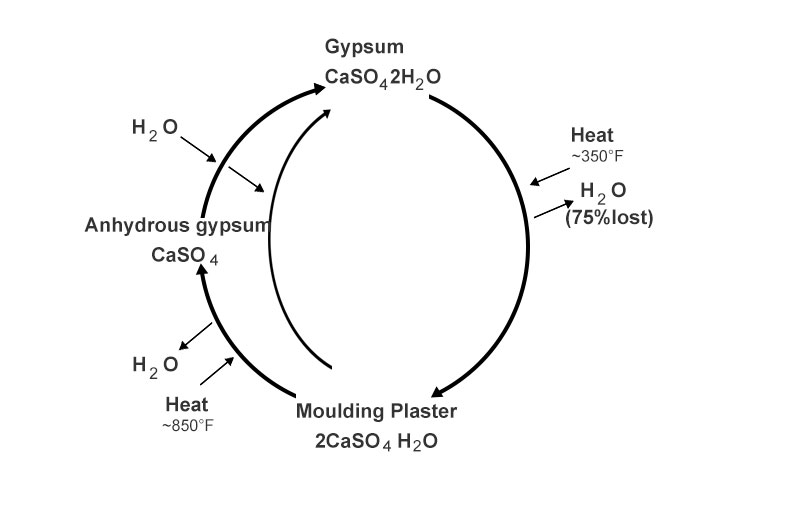
conversion of raw gypsum, plaster of Paris and anhydrite
Of course, plaster of Paris can also be made by reheating and dehydrating the hardened gypsum, but when the plaster is heated to 850° F, all the crystal water will lose and become anhydrous calcium sulfate CaSO4 which is also known as “dead burnt” gypsum or anhydrite by masses. Its theoretical composition of CaO are 41.19%; S03 58.81%; Its Mohs hardness is 3.0 ~ 3.5 and density is 2.8 ~ 3.0 g/cm3. Anhydrous gypsum manifests a distinct crystallization pattern that produces dense, hard sets very useful in casting work.
| Types | Mohs Hardness | Density (g/cm3) |
| Raw Gypsum | 2 | 2.3 |
| Plaster | 2 | 2.55 ~ 2.67 |
| Anhydrite | 3.0 ~ 3.5 | 2.8 ~ 3.0 |
Gypsum is a member of versatile minerals. The value of gypsum has been discovered long ago, with traces of its use being found in the remains of buildings in Israel as early as 7000 BC. Its variety of uses will be explained below.
The common use for society of gypsum is in gypsum board which is also known as gypsum plaster, drywall or wallboard. It is the premier building material for wall, ceiling, and partition systems in residential, institutional and commercial structures and is designed to provide a monolithic surface when joints and fastener heads are covered with a joint treatment system. All modern homes in North America and other developed countries use a large number of gypsum boards as interior walls. The United States is a major consumer of wallboards, with more than 30 billion square feet of annual usage. Today, with over 97% of new homes using gypsum board, it is clearly the interior construction material of choice for the following characteristics.
(1) Rapid setting and hardening.
(2) The volume expands slightly when hardened. Cementitious materials such as lime and cement often shrink when they are hardened, but building gypsum expands slightly (the expansion rate is about 1%), which makes the surface of gypsum products smooth and full, with sharp edges and corners as well as dry without cracking. It lasts so long that the wall will be flawless even after 20 years.
(3) Gypsum plaster is lightweight with large porosity, low apparent density and strength after hardening.
(4) Good thermal insulation and sound absorption.
(5) Good fire resistance. The main component of the gypsum plaster, the crystallization water in the dihydrate gypsum, will evaporate and absorb heat once meets flint. A steam curtain will be formed on the surface of the product, which can effectively prevent the spread of fire.
(6) It has a certain temperature and humidity control properties.
(7) It dries quickly, provides an inert surface for paints and chemicals, and you can apply paint within three days of completing the application easily.

gypsum using as wallboard
Gypsum powder is used as soil conditioner and fertilizer in agriculture. It can be used to alter the soil structure affected by heavy traffic, flooding, overplanting, or over-weathering. It has the ability to improve acidic soils. It provides plants in land with nutrients by providing sulfur and calcium. Calcium helps absorb nutrients from the roots and sulfur increased crop yields. Gypsum powder is particularly beneficial for corn and soybeans, which need large amounts of sulfate in the soil. Gypsum, a sulfate mineral, which has an affinity for water molecules, increases the soil’s ability to hold water. It can also reduce crusting, improve water runoff and erosion control, help seedling emergence, increase soil availability and permeability.

gypsum using as soil fertilizer
When we talk about the medical industry, it’s easy to think of plaster molds made of gypsum powder for fixation of fractures, but the use of gypsum powder in the medical industry is far more than that. First, gypsum plaster is a favorite of all dentists. It can be used to fill cavities, make caps and substitutes and molds for constructing dentures. Gypsum powder is cold and has a heat-clearing and detoxifying effect, so it can be used to alleviate the situation of lung and stomach overheating; It can also quench thirst, cure headache and toothache. What’s more, it can also treat burns once mixed with rhizoma coptidis, anemone, indigo. It makes the skin scab quickly, reduce exudation, and prevent infection.
Building gypsum has excellent properties. It is suitable for interior ornamental, usually, it is made into stucco, architectural and decorative products and sculptures in galleries or gardens.
Portland cement or cementitious material can be produced by adding proper activator, which is suitable for the reinforcement of soft soil foundation, wall coating, mechanical model, tunnel support and fiber press plate production.
Gypsum (calcium sulfate) is recognized by the US Food and Drug Administration as a human edible food and can be used as a dietary source of calcium. Gypsum is used to brew beer and it helps to maintain the proper transparency and acidity. As an additive, it helps to regulate acidity and improve the stability and quality of food. It is often added to foods such as ice cream, dairy products, confectionery and so on.

gypsum using as food additive
Latest blog
 By:ADMINAugust 19,2024
By:ADMINAugust 19,2024
 By:AshleyMarch 20,2020
By:AshleyMarch 20,2020
 By:AshleyMarch 14,2020
By:AshleyMarch 14,2020
 By:AshleyDecember 28,2019
By:AshleyDecember 28,2019
Contact
E-mail:vip@sinoftm.com
Address: No.168 Wutong Street, High-Tech Industrial Development Zone of Zhengzhou City, China.
Hot Products
Future Prospect
We would like to cooperate with customers all around the world, and welcome you sincerely !
Inquiry Online 7×24 Online